Dr. Amy Acton claims the Ohio Health Department found five cases in five counties where COVID-19 symptoms were experienced in January.
Acton mentioned serological antibody testing and hinted that contact tracers were involved in investigating the patients’ cases during a press conference Monday, WLWT reported.
Despite her claims, however, Acton said she does not have the specific counties where the patients are from, the station said.
The Washington Examiner reported that six Ohio patients had been identified as having been ill as early as Jan. 7. Two of them, at least, had traveled outside of Ohio, but officials did not know if that was involved in becoming sick.
WKEF reports the first case in Ohio, dating to January, was in the Miami Valley.
That begs the question: Is antibody testing taking place?
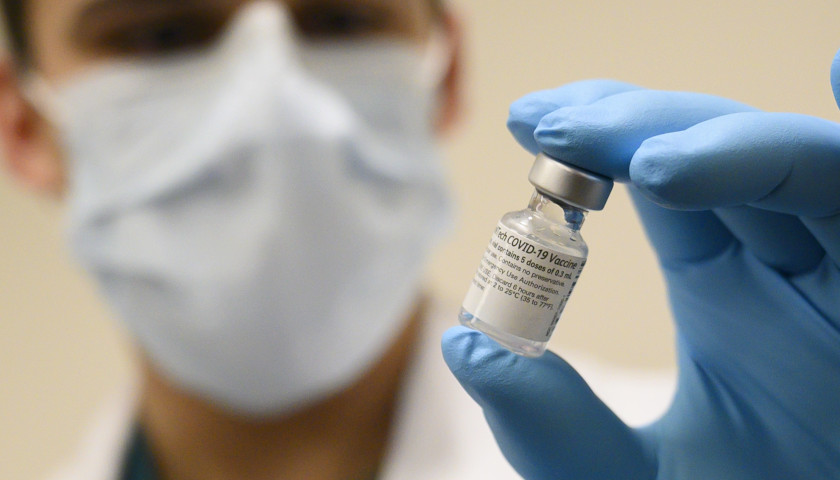
The Health Department on April 13 said some Ohio businesses were buying serological test kits. These tests find antibodies, or proteins, in blood left when the body responds to the virus that causes COVID-19.
The department warns about these tests because many at the time were not approved by the U.S. Food and Drug Administration. The federal government allowed tests to be rolled out before being fully vetted in the rush to address the impact of COVID-19.
These tests detect the immune response to the virus, not the virus itself, and can help healthcare professionals identify individuals who have overcome an infection in the past and developed an immune response. In the future, this may potentially be used to help determine, alongside other clinical data, that patients are no longer susceptible to infection and can return to work, according to the U.S. Food and Drug Administration (FDA).
Ohio Department of Health Director Amy Acton, M.D., MPH, strongly recommends that employers in Ohio review the following information before purchasing or using COVID-19 antibody test kits:
In the early days of an infection when the body’s immune response is still building, antibodies may not be detected. As such, antibody testing is one piece to the puzzle to determine whether employees can return to work. Antibody test results are not the sole answer.
Some firms developing the tests are falsely claiming that their serological tests are approved by the FDA or falsely claiming that they can diagnose COVID-19.
All employers looking to use the tests should ensure that they are buying only antibody tests approved by the FDA for Emergency Use Authorization (EUA). Without the FDA’s EUA approval, there is no way to know if the test kits are valid. Testing companies that are EUA-approved are listed on the FDA website, which is updated daily.
Quest Diagnostics was conducting serological tests in northeast Ohio, Cleveland 19 reported on April 27.
“Antibodies developed by the body in response to a viral infection may provide potential immunity against future infection,” the company said in a news release.
The Health Department on May 9 said it would test 1,200 households randomly for antibodies, Fox 8 said. Households would be notified by mail and could opt-out.
– – –
Jason M. Reynolds has more than 20 years’ experience as a journalist at outlets of all sizes.